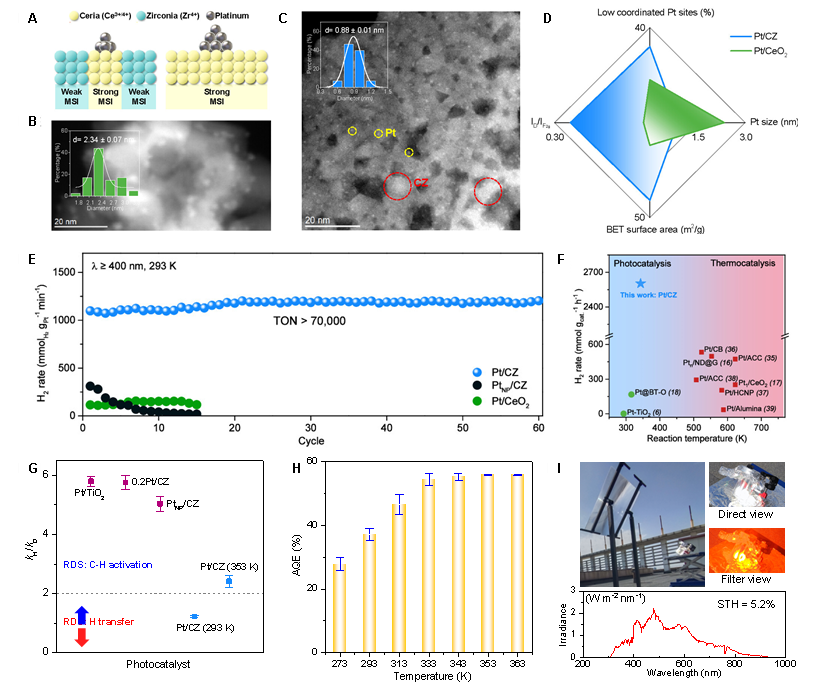
The technology of liquid organic hydrogen carriers presents great promise for large-scale hydrogen storage. Nevertheless, the activation of inert C(sp3)–H bonds in hydrocarbon carriers poses formidable challenges, resulting in a sluggish dehydrogenation process and necessitating high operating temperatures. Here, we break the shackles of C–H bond activation under visible light irradiation by fabricating subnanometer Pt clusters on defective Ce–Zr solid solutions. We achieved an unprecedented hydrogen production rate of 2601 mmol gcat.–1 h–1 (turnover frequency >50,000 molH2 molPt–1 h–1) from cyclohexane, surpassing the most advanced thermo- and photocatalysts. By optimizing the temperature-dominated hydrogen transfer process, achievable by harnessing hitherto wasted infrared light in sunlight, an astonishing 56% apparent quantum efficiency and a 5.2% solar-to-hydrogen efficiency are attained at 353 K. Our research stands as one of the most effective photocatalytic processes to date, holding profound practical significance in the utilization of solar energy and the exploitation of alkanes.
Read more at


